Poster Presentation 1st Asia Pacific Herbert Fleisch Workshop 2025
Coordinated 3D structural and compositional changes between articular and calcified cartilage (#106)
Introduction: Calcified cartilage forms the transitional, mineralised interface between articular cartilage and subchondral bone. It plays a critical role in anchoring the overlying cartilage to the underlying subchondral structures and transmitting mechanical load across the joint1. While degeneration of both tissues is well-documented in osteoarthritis (OA)2, how they remodel relative to each other remains poorly understood. Quantitatively assessing their structural and compositional coupling can provide critical insight in OA pathogenesis, where changes in one tissue can influence the other. In this study, we leverage contrast-enhanced synchrotron-radiation microCT (SR-microCT) to investigate concurrent articular and calcified cartilage changes during OA progression.
Method: Tibiae from male STR/ort (OA, n=16) and CBA/1 (control, n=16) mice from a prior study were used3. Samples were incubated in anionic contrast agent (Hexabrix™) and imaged at 3 μm voxel size using SR-microCT (X02DA-TOMCAT, Swiss Light Source4) within a custom humidity chamber5. Articular and calcified cartilage were segmented using a multi-threshold approach, and 3D structural and attenuation-based compositional metrics were extracted. Statistical analyses were performed using Spearman’s correlation and generalised least squares (GLS) regression models.
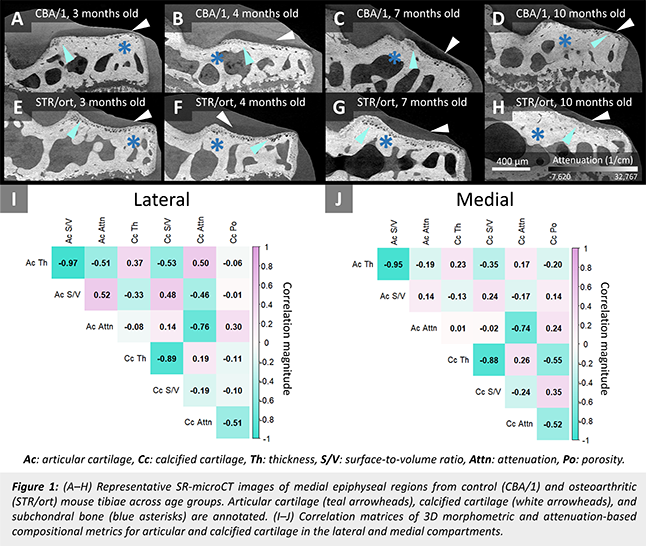
Result: Representative grayscale images are shown in Fig.1 (A-H), and correlation matrices for lateral and medial compartments are presented in Fig.1 (I) and (J), respectively. Articular and calcified cartilage exhibited more coordinated changes in the lateral compartment, particularly for composition (attenuation) and surface irregularity (surface-to-volume ratio), while medial correlations were generally weaker. This may indicate disrupted cross-tissue signalling in OA and is consistent with greater medial tissue damage when observed with confocal microscopy3.
Conclusion: Quantifying structural and compositional coupling in 3D enables the identification of features that reflect cross-tissue interactions—offering a new approach to monitor disease progression and evaluate early intervention targets in OA.
Acknowledgement: We thank the TOMCAT beamline staff for their support. This project received funding from several schemes6,7,8.
- Wang, W. et al. Frontiers in Bioengineering and Biotechnology, 2022, 10, 911281.
- Goldring, S. R. & Goldring, M. B. Nature Reviews Rheumatology, 2016, 12, 632–645.
- Stok, K. S. et al. Bone, 2009, 45, 414–422.
- Stampanoni, M. et al. Developments in X-Ray Tomography V, 2006, vol. 6318.
- Choo, R. J. et al. Review of Scientific Instruments, 2013, 84.
- FWO and F.R.S.-FNRS under the Excellence of Science program (EOS-No.40007553).
- EU Shared-Cost RTD Action (QLK3-CT-2002-02039).
- Beamtime from the Paul Scherrer Institute (20100842, 20110247).