Oral Presentation 1st Asia Pacific Herbert Fleisch Workshop 2025
Osteoclast recycling as a potential mechanism driving rebound bone loss following denosumab withdrawal (#13)
Discontinuation of denosumab (Dmab), results in a rebound increase in osteoclast formation and bone resorption, increasing risk of multiple vertebral fractures. We determined the temporal changes in osteoclast formation following the removal of the RANKL decoy receptor osteoprotegerin (OPG:Fc) in mice, demonstrating early elevations in local and systemic RANKL, and a subsequent overshoot in serum TRAP as osteoclasts rapidly re-formed. More recently, accumulation of osteoclast precursors has been demonstrated to contribute to this rapid rebound in osteoclast formation. Previously, our group characterised a novel osteoclast lineage cell arising from osteoclast fission, osteomorph, which is capable of re-fusing to form osteoclasts. We sought to determine whether OPG:Fc leads to increased osteoclast fission and an accumulation of osteomorphs, providing evidence that osteomorphs may contribute to enhanced osteoclast formation following withdrawal of Dmab.
Treatment of primary murine osteoclasts with OPG:Fc (250ng/ml) for 30 hours led to a 70% reduction in total osteoclast numbers (p<0.01). Interestingly, a reduction in apoptotic osteoclasts (p<0.01), and a 2-fold increase in total cell number were demonstrated (p<0.02). Live-cell imaging of OPG:Fc treated LysM-TdTomato-expressing primary multinucleated osteoclasts over a 30-hour period, demonstrated a significant increase in fission events compared to control (Figure 1a). These data indicate that osteomorphs, in addition to osteoclast precursors, accumulate during anti-RANKL treatment.
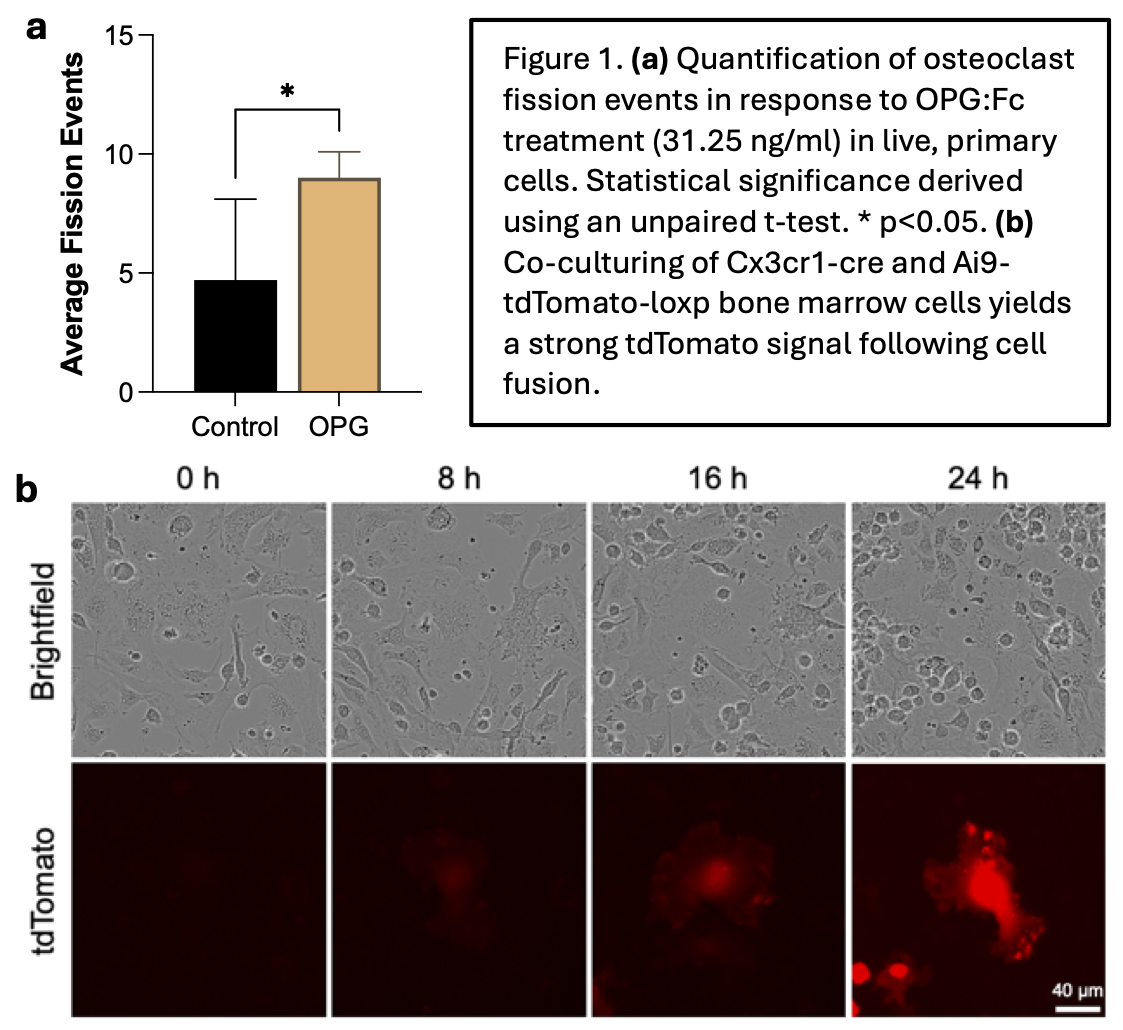
We recently developed a novel osteoclast lineage tracing system whereby, upon fusion of pre-osteoclasts from CX3CR1-cre and Ai9-tdTtomato mice, cytoplasmic tdTomato expression initiates. This system allows, for the first time, fate-tracking of osteoclast fusion and fission products in real time (Figure 1b). We will utilise this system to investigate fission and importantly re-fusion of osteomorphs into osteoclasts to demonstrate their role in bone loss following withdrawal of anti-RANKL treatment. This innovative approach will provide opportunities to interrogate osteomorph biology and develop new insight into advancing sequential therapy approaches for osteoporosis.